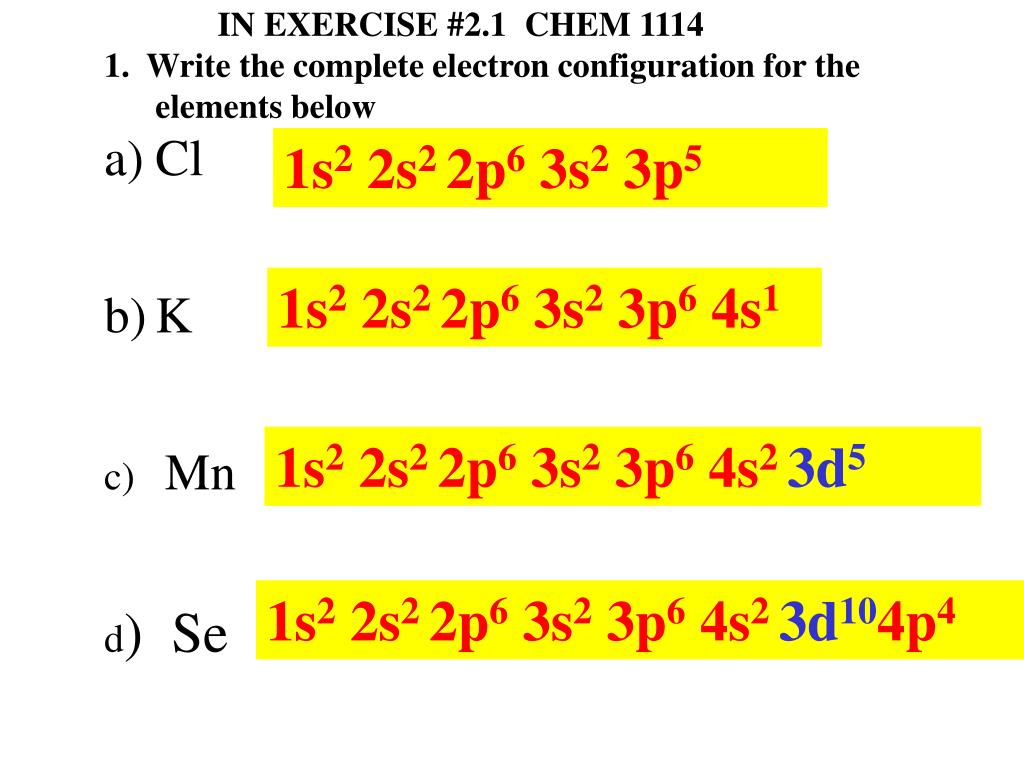
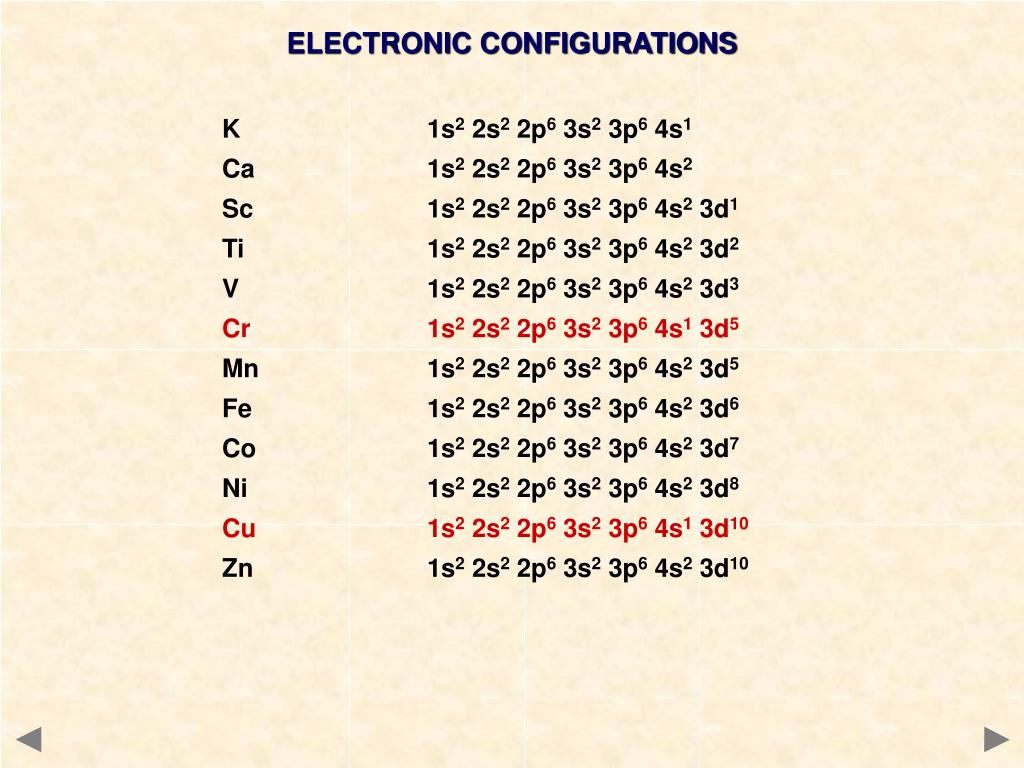
1s2 2s2 2p6 3s2 3p6 4s1 3d5
1s2 2s2 2p6 3s2 3p6 3d5 4s1. In comparison to 3d4 3d9 and 3d10 the configuration is best as half filled fully filled in 3d5 3d10.

What Is Stated In Aufbau S Principle And What Are Its Applications Quora
401 Atomic Number.

. By Staff Writer Last Updated April 14 2020 The electron configuration for manganese is 1s2 2s2 2p6 3s2 3p6 4s2 3d5. With an electron configuration of 1s22s22p63s23p5 or Ne 3s2 3p5 there are a total of 17 electrons so the atomic number is 17 and the. A configuration that remains stable is usually that of a full filled or full filled 3d5 with 3d10 as half filled or full filled or full filled 3d9 with 3d10.
1s2 2s2 2p6 3s2 3p6 4s1 3d 10. Half-filled and fully filled subshell have got extra stability. 1s2 2s2 2p6 3s2 3p1.
The correct answer to the question What is the electron configuration for chromium is letter B or 1s2 2s2 2p6 3s2 3p6 421 3d5. 1s2 2s2 2p6 3s2 3p6 4s1 3d5. 1s2 2s2 2p6 3s2 3p64s2 3d10.
The 1st 2 electron will be in 1s orbital as s-o. 1s2 2s2 2p6 3s2 3p6 4s1 3d10. Tenemos la configuración electrónica de un elemento X.
A 1s2 2s2 2p6 3s2 3p6 4s2 3d4 B 1s2 2s2 2p6 3s2 3p6 4s1 3d5 C 1s2 2s2 2p6 3s2 3p6 3d6 D 1s2 2s2 2p6 3s2 3p6 4s2 4p4. Outermost shell 3d 5. Oxides are binary compounds formed by the reaction of oxygen with other elements.
1s2 2s2 2p6 3s2 3p6 4s1 3d5. The angular quantum number l describes the shape of the orbital. It is a member of group 2 in the periodic table hence it forms a divalent ion Ba2.
It can be shortened to Ar 4s2 3d5 where the Ar represents argon the last element in the third row of the periodic table whose electrons fill every shell prior to the 4s-orbital. However notice that 1s2 2s22p6 3s2 3p6 is the configuration for Argon a noble gas. A diagram showing the.
Add your answer and earn points. 1s2 2s2 2p6 3s2 3p4. Just replace this portion of zincs electron notation with Argons chemical symbol in brackets Ar So zincs electron configuration written in shorthand is Ar4s2 3d10.
Zincs full electron configuration is. The actual electron configuration of Cr is AR 4s1 3d4 and Cu is Ar 4s1 3d10. Cr Chromium as there are 24 electrons in a Cr atom.
The principal quantum number n describes the size of the orbital. 1s2 2s2 2p6 3s2 3p5. Now Letter A or 1s2 2s2 2p6 3s2 3p6 4s2 3d4 is the expected electronic configuration of a chromium since it has 24 electrons.
The atomic number of Cr is 24. What determines orbital shape. 1s2 2s2 2p6 3s2 3p6 4s1 3d5 find group block outermostshell and period 1 See answer Advertisement Advertisement rehmananzil is waiting for your help.
What is the element 1s2 2s2 2p6 3s2. This element belongs to group 1 ie. Up to 10 cash back 1s2 2s2 2p6 3s2 3p6 4s1 3d10.
Which element has the electron configuration of 1s2 2s2 2p6 3s2 3p6 3d5 4s1 1s22s22p63s23p63d54s1To find out the name of the element from 1s2 2s2 2p6 3s2 3. 1s2 2s2 2p6 3s2 3p6 4s2 3d6 OR Ar 4s2 3d6. The elements in coppers group will fill the d orbital at the expense of having a half filled s orbital.
Orbitals for which n 2 are larger than those for which n 1 for example. 1s2 2s2 2p6 3s2 3p6 4s2. 1s2 2s2 2p6 3s2 3p6 4s2 3d7 OR Ar 4s2 3d7.
1s 2 2s 2 2p 6 3s 2 3p. 1s2 2s2 2p6 3s2 3p6 4s1 3d5 Instead of it being 4s2 3d4 it is 4s1 3d5 This is because there is one electron in each orbital of each 4s1 and 3d5 and so it is more stable than having 1 electron less in the 3d4. Therefore one of the 4s2 electrons jumps to the 3d5 so that it is half-filled see video below.
1s2 2s2 2p6 3s2 3p6 4s1 3d5. Which element has the electron configuration 1s22s22p63s1. Which is the correct ground-state electron configuration for a chromium atom Cr.
1s2 2s2 2p6 3s2 3p6 4s2 3d5 Go to the Top of. 1s2 2s2 2p6 3s2 3p6 4s1 3d 10. 1s2 2s2 2p6 3s2 3p6 4s1 que elemento es 2 Ver respuestas Publicidad Publicidad lumar173 lumar173 El elemento cuya configuración electrónica es 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ Z19 es el Potasio Símbolo.
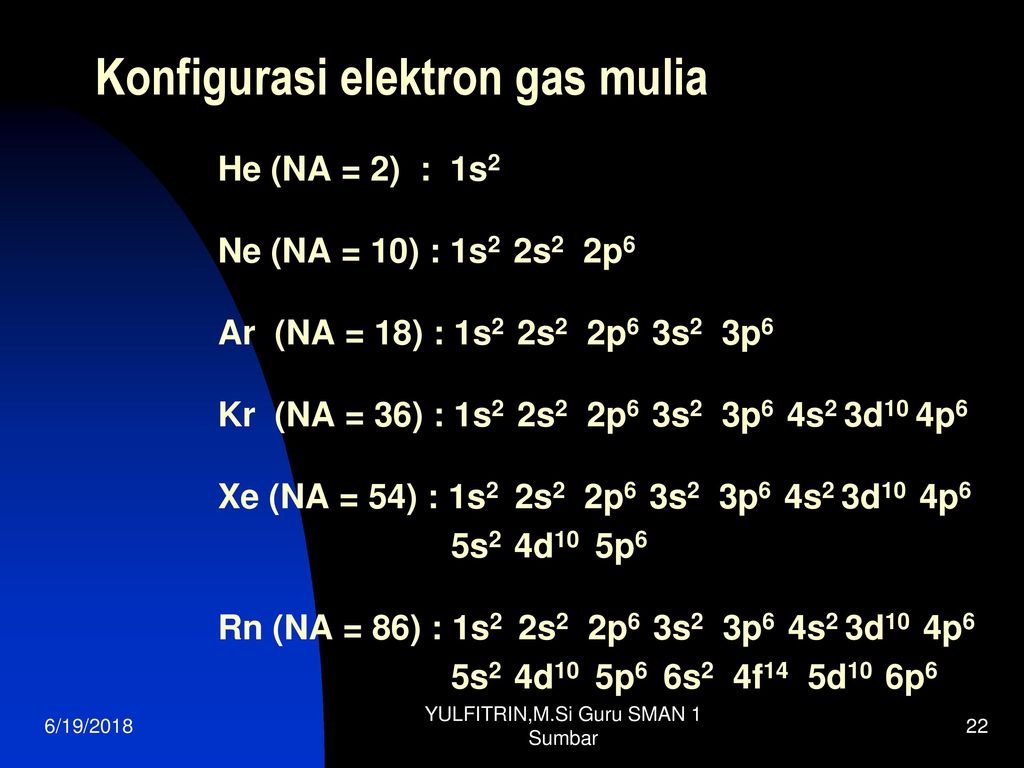
1s2 2s2 2p6 3s2 3p6 4s2 3d5 OR Ar 4s2 3d5. 1s2 2s2 2p6 3s2. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 OR Xe 6s2 4f14 5d10 6p6.
Alkali metals group and metallic oxides are basic. Which element has the electron configuration of 1s22s22p63s23p5 Now you have to look in the periodic table for element number 17 which is Cl. The element Barium has electronic configuration.
1s2 2s2 2p6 3s2 3p6 4s1 3d5. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 5 is the electronic configuration of a. This is because this configuration is more stable than a full s orbital and d orbital with 910 spots filled.
Therefore if the u. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2. 1s2 2s2 2p6 3s2 3p6 4s2.
1s2 2s2 2p6 3s2 3p6 4s1 Go to the Top of the page. Christmas bulbs Electron configuration Christmas ornaments. PLEASE DO RATE IF THIS HELPED.
To figure this out the element with the electron config of we first count the electrons. For the Cr 2 ion we remove one electron from 4s1 and one from the 3d5 leaving us with. Ca Name of Element.
This give us the correct configuration of. An Element Having Configuration 1s2 2s2 2p6 3s2 3p6 4s1 Will Form An element having configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 will form. Those are the small number placed at the top after the letters.
Alkaline Earth Metals. Calcium Atomic Weight. THANKS 1s2 2s2 2p6 3s2 3p6 4s1 3d5 is the elctronic configuration of d.
Cr Expert Answer 100 3 ratings PS. 1s2 2s2 2p6 3s2 3p6 4s1 3d5 OR Ar 4s1 3d5. Answer- The electron configuration 1s2 2s2 2p6 3s2 3p6 4s1 3d5 corresponds to the Cr Chromium.
Due to symmetrical distribution of electrons. An asymmetrical electron density distribution as well as greater transitions energies are to blame.

Which Element Has The Electron Configuration Of 1s2 2s2 2p6 3s2 3p6 3d5 4s1 Youtube
Ppt N 1 Powerpoint Presentation Free Download Id 2351497

Suatu Unsur Memiliki Konfigurasi Elektron 1s2 2s2 2p6 3s2 3p6 4s1 3d5 Unsur Tersebut Dalam Brainly Co Id

Solved Which Electron Configuration S Islare Correct I Cr Atom 1s2 2s2 2p6 3s2 3p6 4s 3d5 Ii Cu Atom Is2 2s2 2p6 3s2 3p6 4s1 3d10 Iii Fe2 Ion Is2 2s2 2p6 3s2 3p6 4s 3d5

Electron Configurations Of Elements Which Are Exceptions To The Rule Pdf Atoms Transition Metals
Struktur Atom Dan Sistem Periodik Unsur Ppt Download
Ppt An Introduction To Transition Metal Chemistry Powerpoint Presentation Id 338679

Suatu Unsur Memiliki Konfigurasi Elektron 1s2 2s2 2p6 3s2 3p6 4s1 3d5 Unsur Tersebut Dalam Brainly Co Id

Which Element Has The Electron Configuration Of 1s2 2s2 2p6 3s2 3p6 3d5 4s1 Youtube

Order Of Filling Orbitals Ppt Video Online Download

Penjelasan Konfigurasi Elektron Dalam Atom Terlengkap Ilmu Kimia Untuk Indonesia

Problem Three Atoms Have The Following Electron Configurations A 1s2 252 2p6 3s2 3p1 B 1s2 Homeworklib
Suatu Unsur Memiliki Notasi Unsur 29 X Konfiguras

Which Element Has The Electron Configuration Of 1s2 2s2 2p6 3s2 3p6 4s1 Youtube

Question What Element Has The Electron Configuration Of 1s2 2s2 2p6 3s2 3p6 4s2 3d7 Excelgreentechnology Com

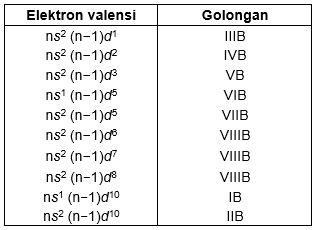
Diketahui Konfigurasi Elektron X Z 28 1s2 2s2 2p6 3s2 3p6 3d8 4s2 Letak Unsur X Dalam Sistem Brainly Co Id


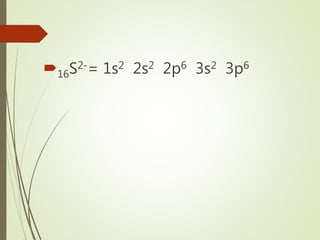
Comments
Post a Comment